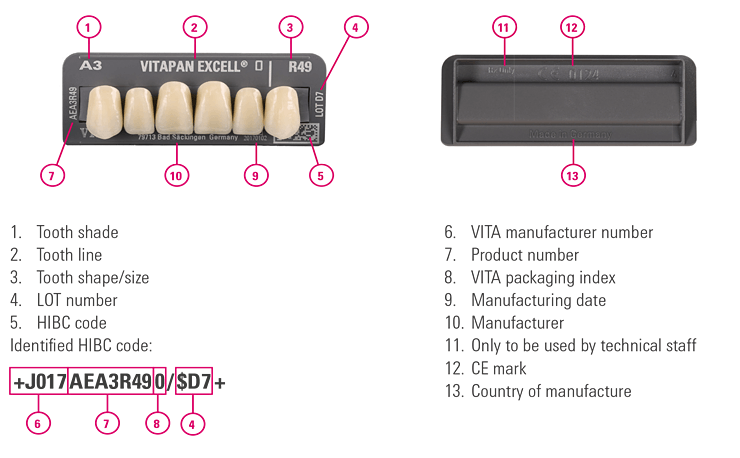
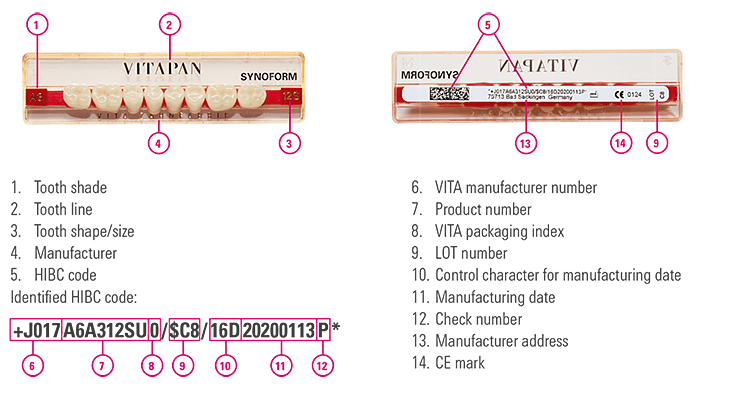
To facilitate documentation, we recommend using a documentation software. Using a barcode reader, all UDI-compliant HIBC information on the medical devices can be scanned, transferred to the documentation soft-ware and saved.
Manual documentation of the product number and batch to ensure sufficient traceability, can also be carried out without a barcode reader.
We do not attach adhesive labels, as this is not a sustainable solution and would require management of vast quantities of labels, given the large number of components.
Declarations of conformity
You can conveniently download and manage declarations of conformity in our MyVITA online portal.
Simply register at www.vita-zahnfabrik.com/MyVITA-Register
In the "Declarations of conformity" section, you can access the service "Automatic updates for your declarations of conformity." If requested, your selected VITA declarations of conformity are updated automatically. You will also be informed via email when a new version is available.
 It appears that you are currently in USA.
It appears that you are currently in USA.